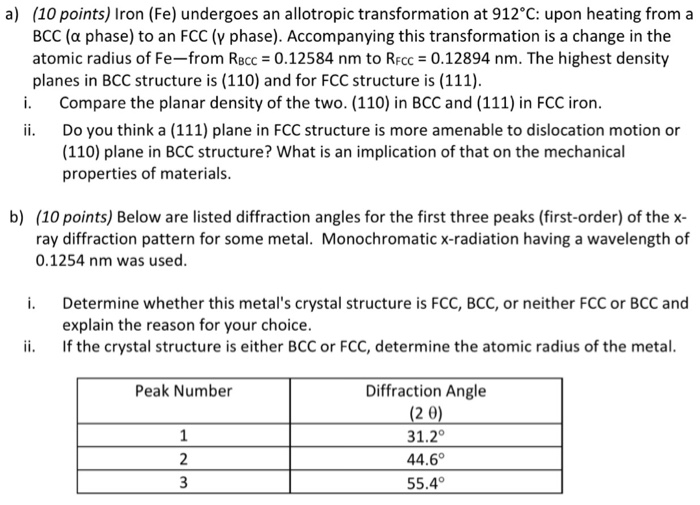
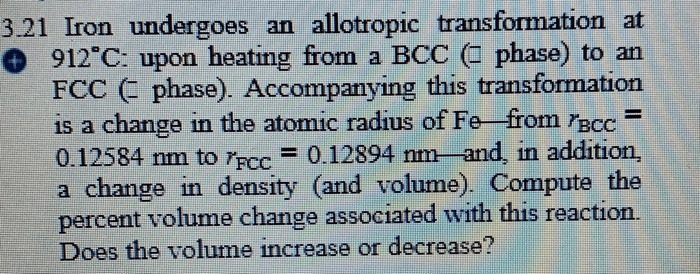
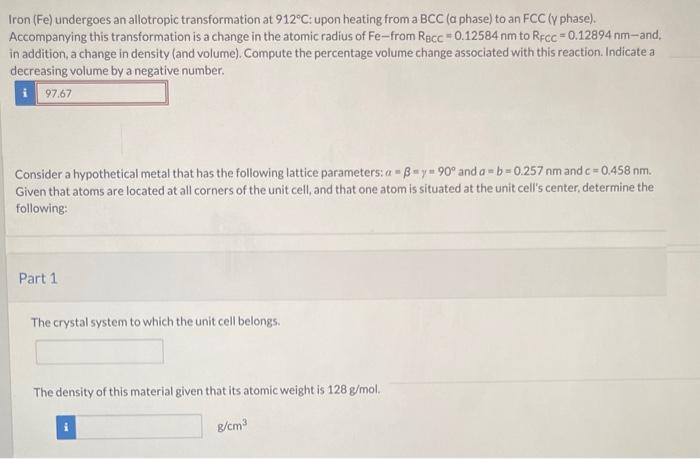
Iron undergoes an allotropic transformation at 912°C, a fascinating phenomenon that marks a profound change in the material’s atomic structure. This transformation holds immense significance in materials science, as it dramatically alters the properties of iron, paving the way for a wide range of applications.
Join us as we delve into the intricacies of this remarkable process, exploring its scientific underpinnings, practical implications, and historical significance.
As iron reaches 912°C, it undergoes a transformation from a body-centered cubic (BCC) structure to a face-centered cubic (FCC) structure. This change in crystal structure results in significant alterations in the material’s physical and chemical properties, including its strength, hardness, and magnetic susceptibility.
Understanding the factors that influence this transformation and the resulting changes in iron’s properties is crucial for harnessing its full potential in various industrial applications.
Allotropic Transformation of Iron
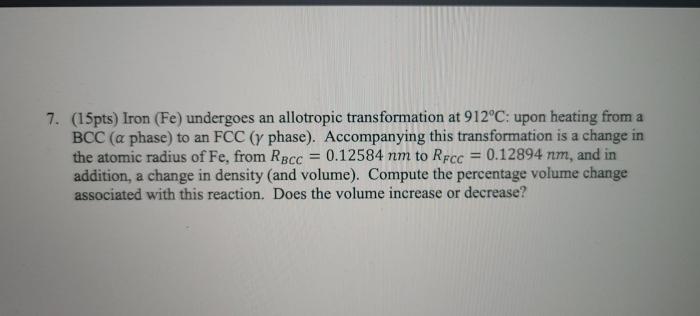
Allotropic transformation refers to the change in the crystal structure of a solid material without any change in its chemical composition. Iron undergoes an allotropic transformation at 912°C, which significantly affects its properties.
Description of the Transformation
At 912°C, iron transforms from its body-centered cubic (BCC) structure, known as ferrite (α-Fe), to a face-centered cubic (FCC) structure, known as austenite (γ-Fe). This transformation is reversible, and upon cooling, austenite transforms back to ferrite below 912°C.
Properties of Iron Before and After Transformation
Ferrite (α-Fe)
- BCC crystal structure
- Strong and ductile
- Magnetic below 768°C (Curie temperature)
- Lower hardness and strength than austenite
Austenite (γ-Fe)
- FCC crystal structure
- More ductile and harder than ferrite
- Non-magnetic
- Higher strength and hardness than ferrite
Factors Influencing the Transformation
- Temperature:The allotropic transformation occurs at 912°C at atmospheric pressure.
- Pressure:High pressure can shift the transformation temperature.
- Alloying Elements:Alloying elements such as carbon, manganese, and nickel can alter the transformation temperature and the resulting microstructure.
Applications of Iron Allotropy, Iron undergoes an allotropic transformation at 912
- Heat Treatment:The allotropic transformation is used in heat treatment processes to control the properties of iron-based materials, such as steel.
- Welding and Joining:The transformation is involved in welding and joining processes, where the material is heated above the transformation temperature to achieve a desired microstructure.
- Magnetic Materials:The allotropic transformation is utilized in the production of magnetic materials, as ferrite is magnetic below 768°C.
Historical Significance of Iron Transformation
The allotropic transformation of iron has played a pivotal role in the development of metallurgy and materials science. It has enabled the understanding and utilization of iron for various applications throughout history.
FAQ Guide: Iron Undergoes An Allotropic Transformation At 912
What is an allotropic transformation?
An allotropic transformation is a change in the crystal structure of a material, resulting in a new arrangement of atoms. In the case of iron, it undergoes a transformation from a body-centered cubic (BCC) structure to a face-centered cubic (FCC) structure at 912°C.
How does the allotropic transformation affect the properties of iron?
The transformation significantly alters the physical and chemical properties of iron. The FCC structure is more closely packed than the BCC structure, resulting in increased strength and hardness. Additionally, the FCC structure is non-magnetic, while the BCC structure is magnetic, leading to a change in magnetic properties.
What are the practical applications of iron’s allotropic transformation?
The transformation is utilized in various industrial applications to enhance the properties of iron-based materials. For example, the transformation is employed in heat treatment processes to improve the strength and toughness of steel. It is also used in the production of ductile iron, which has superior strength and ductility compared to gray iron.